EndoGastric Solutions Takes the Stage at DDW, World’s Largest Gathering of GI, Hepatology, and Endoscopy Specialists
Adrian Lobontiu, MD, FACS, Chief Medical Officer of EndoGastric Solutions
Each year, leading researchers and clinicians in the fields of gastroenterology, hepatology, and endoscopy gather at Digestive Disease Week® (DDW®) to share cutting-edge data and gain insights into the latest advances in clinical care. This year the meeting took place in San Diego, May 18–21, marking its 50th anniversary. Approximately 15,000 physicians, researchers, and academics from across the world gathered over four days—networking, participating in educational activities and learning more about advances in diagnostics, therapeutics, and data. DDW 2019 featured 813 original sessions (oral and poster), 4,229 posters, and 291 exhibitor booths.
As a leader in evidence-based, incisionless surgical technology for the treatment of GERD, EndoGastric Solutions® has had a strong presence at DDW for the past several years. At DDW 2019, customers presented at one podium and two poster sessions reporting new data that further support the safety, efficacy, and clinical utility of Transoral Incisionless Fundoplication (TIF® 2.0) procedure performed with the EsophyX® device. The company also organized an educational program highlighting recent advances in the clinical use of TIF 2.0 to improve patient care and outcomes and held a TIF 2.0 User Group Meeting in which clinicians exchanged ideas about the optimum use of TIF 2.0 in clinical practice.
The educational program, titled “Shaping the Future of GERD: Now is the Time for the TIF 2.0 Procedure,” was held in the DDW Product Theater and featured three presentations and a question and answer session with the presenters.
- Barham Abu Dayyeh, MD, MPH, FASGE, Director of Advanced Endoscopy, Consultant, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Mayo Clinic, Rochester, MN reviewed recent data in Gastrointestinal Endoscopy that described the evolution of and improvements in TIF techniques and the EsophyX technology. He also outlined a framework to select patients for TIF 2.0 based on medical and anatomical considerations. He emphasized that improved endoscopic interventions and careful patient selection can bridge gaps in treatment and target the underlying cause of gastroesophageal reflux disease (GERD).
- Glenn Ihde, MD, General Surgeon, Matagorda Medical Group in Bay City, Texas, reviewed the results of a retrospective study that evaluated subjective and objective effects of TIF 2.0 combined with hiatal hernia repair in patients with GERD. The study, which was published earlier this year in the Journal of the Society of Laparoscopic Surgeons, showed that the combined procedure significantly improved outcomes in GERD patients with respect to subjective assessments (GERD Health-Related Quality of Life [HRQL] questionnaire and Reflux Symptom Index [RSI] measurements) and objective pH scores. Importantly, mean pH scores improved from 35.3 to 10.9 (p < 0.001) following TIF 2.0 in combination with hiatal hernia repair. Notably, subjects with intact hiatal hernia repairs showed a 95% normalization of pH, reinforcing the importance of accurately assessing all anatomical components of the antireflux barrier.
- Kenneth Chang, MD, FACG, FASGE, Professor and Chief, Division of Gastroenterology and Executive Director of the H.H. Chao Comprehensive Digestive Disease Center, UCI School of Medicine Irvine, CA, described his personal experience with state-of-the-art endoscopic foregut surgery and interventions, which had been published in the first 2019 issue of World Journal of Gastroenterology. He set forth a vision for the growing role of interventional endoscopy in enabling minimally invasive alternatives to open or laparoscopic surgery that provide durable results for a variety of GI conditions.
On Monday, May 20, the TIF 2.0 procedure was featured during a podium presentation by the American Society for Gastrointestinal Endoscopy (ASGE) during the New Technology Endoscopic Advancements Session, titled Efficacy and Patient Satisfaction of Single-Session Intraoperative Transoral Incisionless Fundoplication and Laparoscopic Hernia Repair. The study was designed to assess patient satisfaction, symptom resolution, safety, and proton pump inhibitor (PPI) use following the concomitant TIF 2.0 procedure among 33 patients with large hiatal hernias (>2cm). Data was collected pre- and post-procedure from June 2015 to June 2018. Key findings included: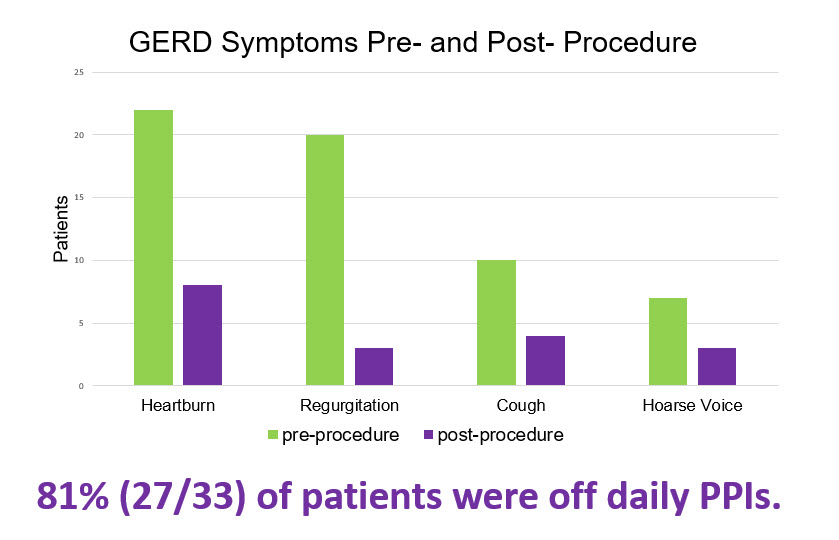
- 94% (31/33) of patients reported 75% or greater satisfaction with the procedure and outcomes
- 81% (27/33) of all patients discontinued the use of daily PPIs due to symptom reduction
The authors concluded that the concomitant TIF 2.0 procedure is an effective therapeutic option for patients with severe refractory GERD and large hiatal hernias.

Dr. Yasser Salem of South Coast Global Medical Center was recognized for treating his 300th TIF patient
Also on May 20, nearly 80 attendees participated in the EndoGastric Solutions User Group Dinner, which included a number of key opinion leaders who have played critical roles in advancing the use and adoption of the TIF 2.0 procedure. Attendees gained insight into the technical evolution of the TIF 2.0 procedure –from Endoluminal Fundoplication (ELF) through TIF 2.0, and reviewed the latest data supporting the use of TIF 2.0 as a stand-alone procedure, in combination with hiatal hernia repair, and after peroral endoscopic myotomy (POEM) to improve outcomes for GERD patients. The discussion at the User Group Dinner also emphasized the importance of collaboration and communication between specialist GIs and surgeons in effectively co-managing patients with GERD and aligning diagnostic processes, interventional procedure logistics, and reimbursement requirements to achieve optimal outcomes. Dr. Yasser Salem of Orange County, CA was recognized for having treated his 300th TIF patient.
Dr. Bindiya Shah Easton Hospital presenting on the effectiveness of the TIF 2.0 procedure in improving both typical and atypical symptoms of GERD at DDW 2019
A growing body of clinical data supports the safety and efficacy of TIF 2.0 in the treatment of GERD, and two additional studies of TIF 2.0 were presented at DDW 2019. One study, titled “Transoral Incisionless Fundoplication: Effectiveness in Improving Typical and Atypical Symptoms of Gastroesophageal Reflux Disease Incompletely Controlled with Medical Therapy,” evaluated the efficacy of TIF 2.0 in controlling heartburn, regurgitation, dysphagia, or atypical symptoms in patients with chronic refractory GERD. The study, which was conducted in 41 patients and utilized the GERD-HRQL and RSI scores and also assessed the use of anti-acid medications, found significant improvements in heartburn, dysphagia, and regurgitation score pre- and post-TIF (p < 0.001, 0.006 and 0.001, respectively) at a median follow-up of nine months. GERD-HRQL and RSI scores also improved significantly following TIF (p < 0.001 for each assessment). The study also found that, at short-term follow-up, TIF 2.0 eliminates the need for PPI medications in a majority of patients. These results support the use of TIF 2.0 as an alternative to conventional medical therapy for appropriately selected patients.

Olaya Brewer Gutierrez, a gastroenterologist in the Department of Gastroenterology at the Johns Hopkins University School of Medicine
Taken together, our various activities at DDW 2019 underscore the growth of the TIF 2.0 community, and the increasing awareness and clinical use of this important treatment option. I’d like to take this opportunity to thank all those who participated in our events and to acknowledge our clinical collaborators and the patients who participate in the clinical trials that are so important to advancing evidence-based approaches to treating GERD and other gastrointestinal disorders. I would also like to congratulate Olaya Brewer Gutierrez, a gastroenterologist in the Department of Gastroenterology at the Johns Hopkins University School of Medicine, on her receipt of the best video award during the American Society of Gastroenterology (ASGE) Video Plenary Session III. As everyone at EndoGastric Solutions continues our efforts to improve the care and outcomes for patients with gastrointestinal diseases, we are already looking forward to sharing our advances at DDW 2020.