– Developed in partnership with DoctorPlan®, the app utilizes data to connect patients with expert clinicians as they jointly develop individualized plans for GERD treatment –
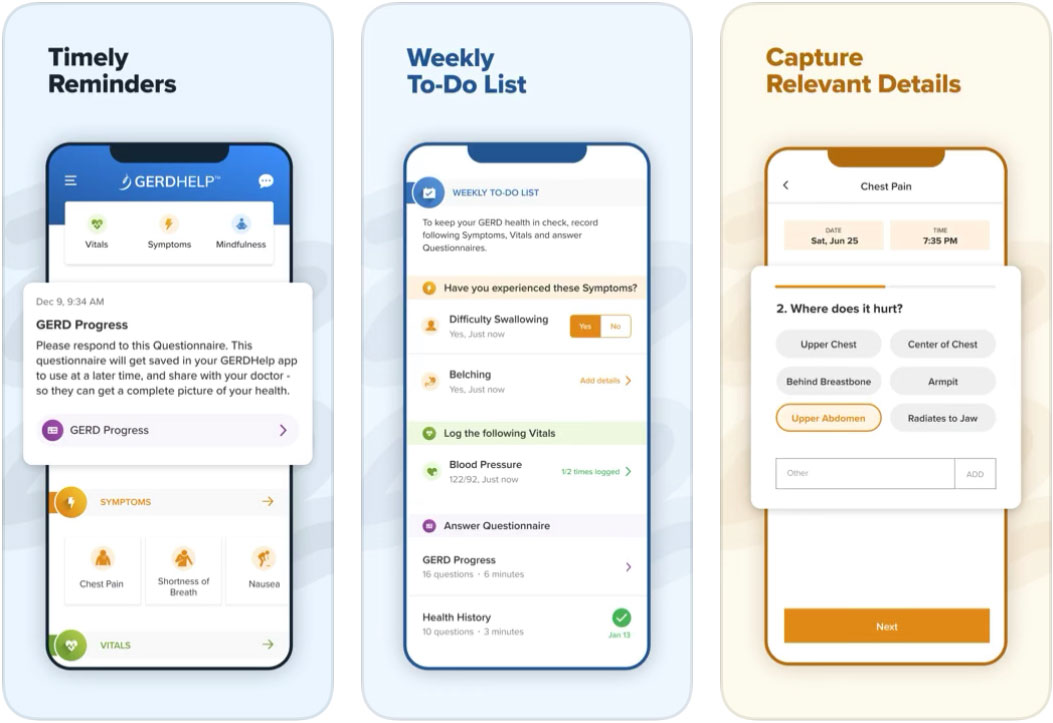
REDMOND, Wash – June 23, 2021 – EndoGastric Solutions® today released the GERDHelp™ mobile application that will virtually guide gastroesophageal reflux disease (GERD) sufferers through an individualized treatment pathway providing education and customized support along the patient journey. Patients can easily record GERD-related symptoms, as well as their frequency, severity, and patterns to better understand the impact GERD is having on their quality of life. This patient data collected between office visits provides the clinician of choice with a comprehensive picture of the disease to tailor/customize individualized care plans.
“GERD is a spectrum disease that plagues millions of Americans with frequent symptoms, both objective and subjective, requiring an individualized treatment plan,” said Rohit Agarwal, co-Founder and CEO of DoctorPlan®. “By collecting patient data between visits, the GERDHelp app keeps patients informed and engaged throughout their journey and helps guide clinicians to deliver better patient care.”

“Many sufferers take proton pump inhibitors (PPI’s) or H2 blockers to manage their symptoms. However, more than 30% of patients have GERD that is refractory to medications and may have an anatomical problem requiring interventional treatment,” said Peter Janu, MD, a general surgeon at Fox Valley Surgical Associates in Appleton, Wisconsin. “The app data allows me to better identify those patients sooner, and then tailor a treatment plan specific to each individual’s position along the disease spectrum.”
GERDHelp mobile app key features include:
- A robust GERD article library to educate and inform patients
- Symptoms, medication and vitals tracking
- GERD lifestyle support including diet and exercise guidance and mindfulness lessons
- Food, water and bowel movement journaling
- Secure two-way communication with GERDHelp nurses
- Diagnostic pathway guidance and treatment-specific FAQs
- Automated follow-up
“The combination of pharmacological options that do not address anatomical deficiencies and the long-term de novo side effects associated with traditional anti-reflux surgeries have created a significant treatment gap and left GERD sufferers without many options,” said Skip Baldino, President and CEO of EndoGastric Solutions. “The GERDHelp app will provide patients with helpful tools and education that will enable them to discover alternative, less invasive and durable treatment options like the TIF® 2.0 procedure using the EsophyX® device, which yields reproducible outcomes without the long-term side effects.”
EndoGastric Solutions is currently enrolling patients for the app’s release. The GERDHelp mobile app is available on iOS® and Android™ devices.
NP02568-01A
