Press Releases
EndoGastric Solutions Announces New Data at Digestive Disease Week 2018 Supporting the Durability and Efficacy of TIF 2.0 Procedure for GERD Patients
TIF 2.0 procedure provides improved quality of life and elimination of daily PPI use for up to 10 years in patients with symptomatic GERD; TIF 2.0 procedure combined with laparoscopic hiatal hernia repair is a safe and effective option for patients with severe GERD...
EndoGastric Solutions Announces Publication of Meta-Analysis Further Validating TIF 2.0 procedure as an Appropriate Treatment Option for Refractory GERD Patients
TIF Procedure offers excellent short and long-term relief for the majority of chronic GERD patients. REDMOND, Wash – EndoGastric® Solutions today announced the publication of a meta-analysis supporting the clinical utility of Transoral Incisionless Fundoplication...
EndoGastric Solutions Announces Publication of 5 Year TEMPO Data Confirming Lasting Improvement of GERD Symptoms with TIF 2.0 Procedure
Cost comparison analysis indicates significantly lower healthcare utilization by TIF procedure patients REMOND, Wash. – EndoGastric® Solutions today announced that Surgical Innovation: Advances in Minimally Invasive Surgical Science, Technology, and Training has...
EndoGastric Solutions announces 100 Million Lives Now Covered for Transoral Incisionless Fundoplication Procedure
Policy decisions by federal and commercial payers have expanded coverage for the TIF 2.0 procedure in 2018, extending access to effective long-term control of acid reflux symptoms REDMOND, Wash. – Federal and commercial payer plans responsible for over 100 million...
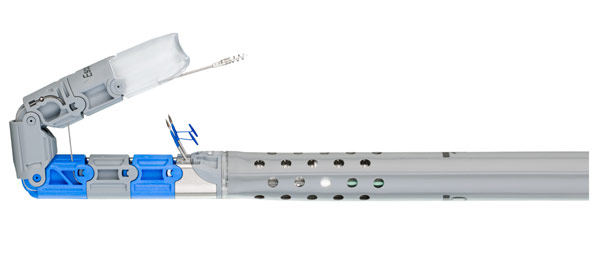
EndoGastric® Solutions Debuts EsophyX® Z+ Device for Transoral Incisionless Fundoplication (TIF) Procedures
New EsophyX Z+ device supports TIF 2.0 procedure with feature set of EsophyX Z device and benefits of adult sized endoscopes. REDMOND, Wash. – EsophyX® Z+ device offers expanded endoscope compatibility and joins EndoGastric Solutions’® portfolio of specialized...
EndoGastric® Solutions Announces Further Expansion of Medicare Reimbursement for Transoral Incisionless Fundoplication (TIF®) 2.0 Procedure
Expanded Medicare coverage and update to facility reimbursement increases patient access to non-surgical alternative for long-term control of acid reflux symptoms REDMOND, Wash. (November 14, 2017) - Medicare beneficiaries in 23 additional states have been granted...
EndoGastric® Solutions Announces Expanded Medicare Reimbursement for Transoral Incisionless Fundoplication (TIF®) Procedure
Effective alternative for long-term control of acid reflux symptoms will now be available to over 60% of Medicare patients REDMOND, Wash. - Medicare beneficiaries in 10 Midwestern and Northeastern states will soon have reimbursed access to the Transoral Incisionless...
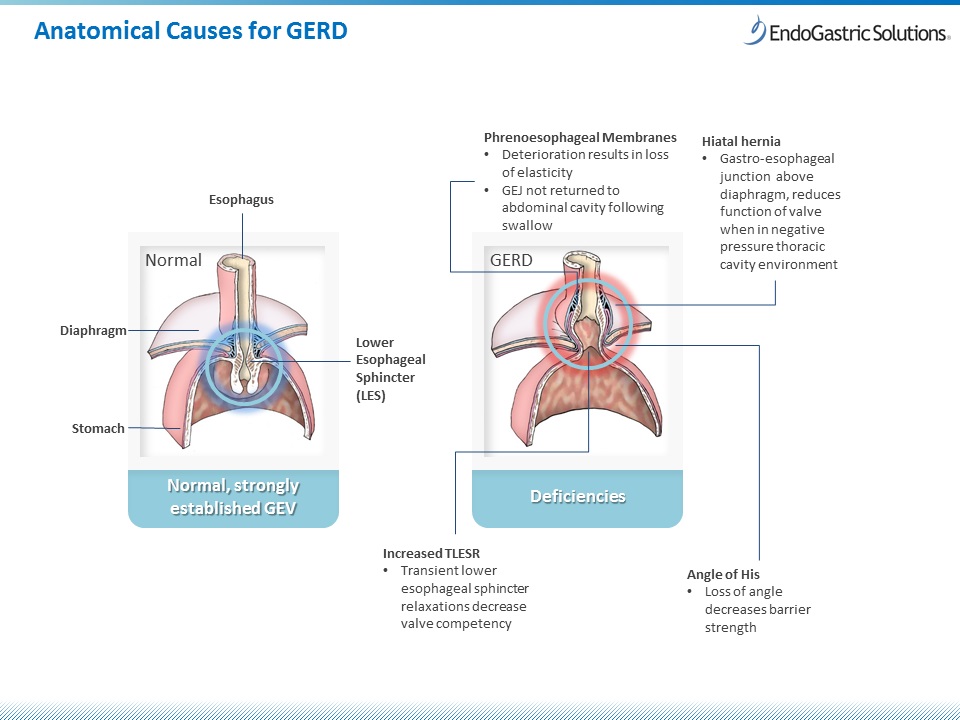
EndoGastric Solutions Announces 12-month Data Validating Hiatal Hernia Repair Prior to Transoral Incisionless Fundoplication (TIF®) as a Durable GERD Treatment Option
Study participants saw 80 percent improvement for heartburn and regurgitation one-year post procedure when hiatal hernia and gastroesophageal valve were repaired sequentially REDMOND, Wash. - EndoGastric Solutions® (EGS), a leader in incisionless procedural therapy...
EndoGastric Solutions announces Coverage Verification by HealthNet for TIF® 2.0 Procedure with EsophyX® Device
Reflux sufferers in Arizona, California, Oregon and Washington now have access to less invasive reflux treatment option.