EsophyX Technology
There are many patients who have tried dietary and lifestyle changes to treat their GERD. However, the outcomes may not have had a sufficient impact on their long-term symptoms. Similarly, some patients are dissatisfied with the available over the counter and prescribed medications to treat their GERD symptoms. Some patients are also hesitant to undergo conventional antireflux surgery due to the incisions required, associated risks, and undesirable side-effects.
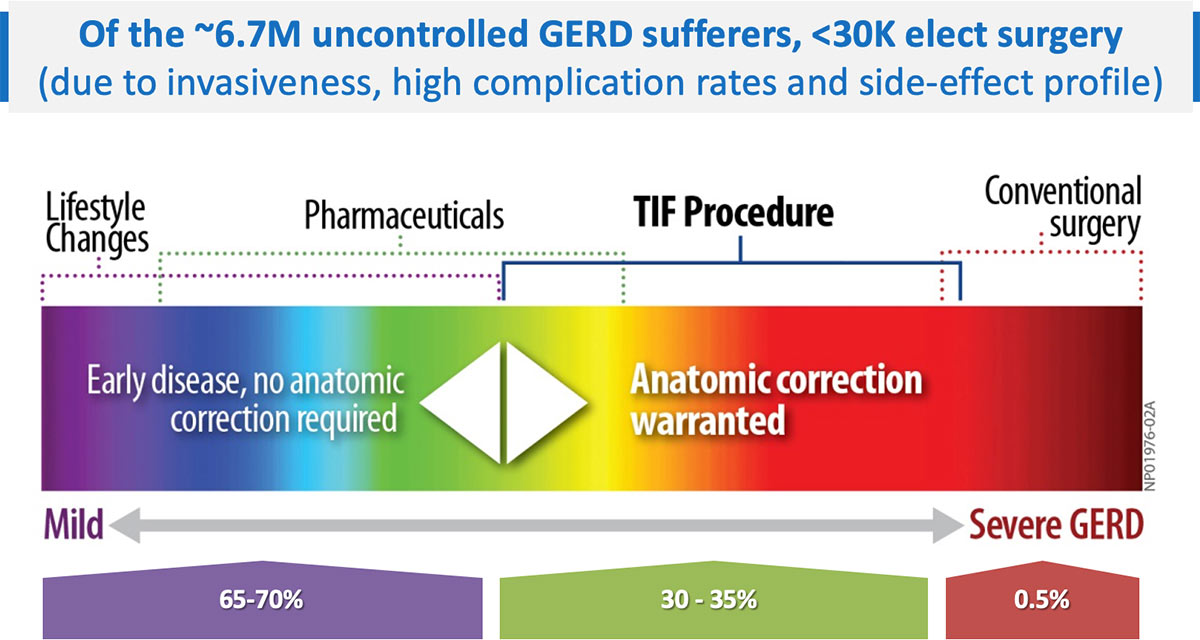
The TIF procedure bridges the treatment gap between medication and more invasive surgical solutions. The incisionless procedure is performed entirely through the patient’s mouth (transorally); it rebuilds the antireflux value and restores the body’s natural protection against reflux.
The advantages of this approach over laparoscopic or open surgery include an improved safety profile, lower complication rates, shorter hospital stays, faster recovery, reduced patient discomfort, and no scars, resulting in higher patient satisfaction. Patients are typically able to return home and back to normal activities the day following the procedure.
Read more about the EsophyX® Device and SerosaFuse® Fasteners technology which enables the TIF procedure’s incisionless approach.
Reference:
Reavis KM, Perry KA. Expert Rev Med Devices. 2014 Jul;11(4), 341-50.
Subramanian, CR and Triadafilopoulos G. Refractory gastroesophageal reflux disease. Gastroenterol. Rep. (2015) 3 (1): 4153.