Press Releases
Merit Medical Announces Asset Purchase Agreement with EndoGastric Solutions, Inc.
Asset acquisition expands Merit’s endoscopy portfolio with a minimally invasive solution for patients suffering from chronic gastroesophageal reflux disease (GERD). Asset acquisition projected to add approximately $30 million of revenue, on an annualized basis, in...
EndoGastric Solutions, Inc.® Expands Executive Leadership Team with Key Industry Veterans
EndoGastric Solutions, Inc.® (EGS) today announced the addition of two members to the executive leadership team. Brett Reynolds is appointed Chief Financial Officer, and Sarah Lyon joins as Vice President of Healthcare Economics, Reimbursement, and Policy.
EndoGastric Solutions, Inc.® Announces CEO Transition and $18M Financing to Expand Commercialization
— Darin Hammers appointed Chief Executive Officer — — EGS closes on $18M financing to further drive growth and adoption of the Transoral Incisionless Fundoplication (TIF) procedure — REDMOND, Wash. — December 5, 2022 — EndoGastric Solutions, Inc.® (EGS) announced that...
EndoGastric Solutions, Inc.® Develops Reflux-Friendly Cookbook to Promote GERD Awareness Week
REDMOND, Wash.--(BUSINESS WIRE)--EndoGastric Solutions, Inc.® today announced the companies’ national campaign, “GERD Gourmet; Recipes for Reflux,” supporting GERD Awareness Week, November 20th-26th. The campaign aims to provide healthy, easy-to-follow reflux-friendly...
EndoGastric Solutions, Inc.® Announces TIF® Procedure Included in New American Gastroenterology Association (AGA) Clinical Practice Update and American College of Gastroenterology (ACG) Guideline
— Leading Gastroenterology Experts Recommend Transoral Incisionless Fundoplication (TIF) for Select Patients with Gastroesophageal Reflux Disease (GERD) — REDMOND, Wa.--(BUSINESS WIRE)--EndoGastric Solutions, Inc.® today announced that the American Gastroenterology...
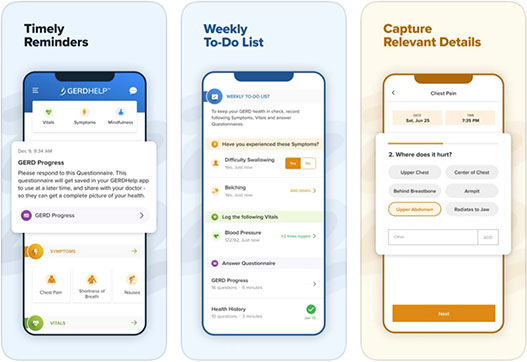
GERDHelp™ Mobile App to Assist GERD Patients in Navigating Their Journey With Individualized Education and Support, Provided by EndoGastric Solutions®
- Developed in partnership with DoctorPlan®, the app utilizes data to connect patients with expert clinicians as they jointly develop individualized plans for GERD treatment - REDMOND, Wash – June 23, 2021 – EndoGastric Solutions® today released the GERDHelp™...
New Publication on TIF 2.0 in Therapeutic Advances in Gastroenterology Provides a Roadmap as Surgeons and Gastroenterologists Partner to Use the Procedure to Achieve Optimal Outcomes for Patients with Gastroesophageal Reflux Disease (GERD)
Continued evolution of the EsophyX technology in TIF performed consecutively after a hiatal hernia repair, in the same anesthesia setting, brings long-term GERD relief to a broader spectrum of patients REDMOND, Wash – June 17, 2020 – EndoGastric Solutions® today...
EndoGastric Solutions Announces New Data Confirming the Efficacy of Consecutive Hiatal Hernia Repair and TIF 2.0 Procedure in Providing Symptom Control for GERD Patients
A 99-patient study confirms that a laparo-endoluminal approach reduces proton pump inhibitor medication use and significantly improves quality-of-life scores REDMOND, Wash – EndoGastric Solutions® today announced the publication of new clinical data confirming that...
EndoGastric Solutions Announces Data Presented at ACG 2018 Provide Additional Validation of the Safety and Clinical Efficacy of TIF 2.0 Procedure for the Treatment of GERD
Amit Sohagia, MD received ACG’s Outstanding Poster Presenter Award for TIF 2.0 procedure poster REDMOND, Wash. – EndoGastric®Solutions today announced the presentation of three posters at the American College of Gastroenterology (ACG) 2018 Annual Scientific Meeting...